Feb. 9, 2021
Researchers unlock the potential of an average MRI scan

Medical imaging is a non-invasive way to look inside the human body, revealing the inner workings of our joints, organs, and brains. It is a powerful diagnostic tool that helps clinicians map how our bodies function, and often more urgently, the dysfunctions that make life much more difficult.
That’s where the work of this year’s T. Chen Fong Postdoctoral Fellowship winners comes in. They’re unlocking the immense potential of medical imaging to increase our understanding of the human brain. All four Cumming School of Medicine postdocs are using magnetic resonance imaging, commonly known as MRI, to study brains at different stages of development, disease progression, or injury recovery.
For these projects, an MRI is just the first step — each scholar is pairing additional technologies or programs with the imaging capabilities of an MRI to better understand and utilize the wealth of data the image provides.
Dr. Tiffany Bell, PhD, Department of Radiology (Supervisor: Dr. Ashley Harris, PhD)
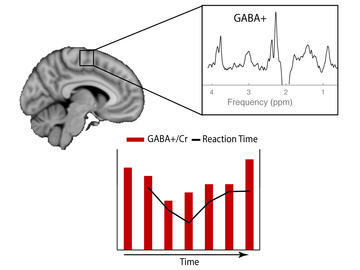
Tiffany Bell is working to create new clinical methods to aid in the diagnosis and treatment of brain injury and disorders. Her work is centred on examining the neurochemicals in the brain that are altered as a result of migraines, concussion, brain injuries, and developmental and mental health disorders.
Bell is studying how neurochemistry presents on a 3T MRI — the hospital standard in Canada — in comparison to how it presents on a much stronger 7T MRI. While a 7T MRI can show a brain’s neurochemistry in greater detail, these machines are not accessible for most clinicians.
By comparing data from the same brain at 3T and 7T, Bell will look for how biomarkers indicating chemical abnormalities show up in 7T data and determine how they correlate to data produced by a 3T MRI. Bell is also developing a method to study how neurochemistry changes while the patient is doing a task, providing a way to determine how altered neurochemistry in the brain relates to behaviour symptoms. Combined, these comparisons will enable her to create a new clinical method for brain injury and disorder diagnosis accessible to all researchers with access to a 3T MRI.
Dr. Bryce Geeraert, PhD, Department of Paediatrics (Dr. Adam Kirton, PhD)
Bryce Geeraert is focused on helping children who have lost or diminished muscle movement as the result of a stroke. He is investigating the use of non-invasive brain stimulation to rehabilitate their motor function, by stimulating the brain using low-intensity focused ultrasound. This technique allows clinicians to target regions of the brain as small as a grain of rice, both at the surface level of the brain and deep within.
Currently, rehabilitative treatments can stimulate only the surface of the brain to improve motor function. Geeraert aims to compare the strengths and weaknesses of low-intensity focused ultrasound to the current gold standard for stimulation. The goal is to prove this new technique is a promising method to improve motor therapies for kids who have had a stroke.
Volunteers will undergo an MRI scan to collect a picture of their brain, which will be used to visualize, target and stimulate motor areas of the brain using ultrasound. A new piece of software developed by Geeraert and team will create heatmaps of the brain that pinpoint how the brain responds to the ultrasound stimulation, and to describe which areas of the brain are responsible for which muscle movement.
Dr. Kathryn Manning, PhD, Department of Radiology (Dr. Catherine Lebel, PhD)
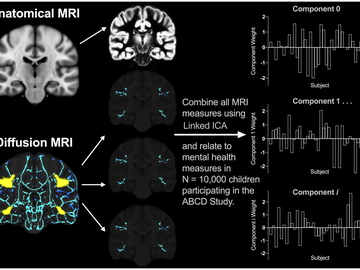
Kathryn Manning is working to identify biomarkers for mental illness in the developing brain. Manning will be using an incredible dataset: a set of progressive MRI scans and clinical assessments of over 10,000 children who will receive bi-annual MRIs beginning at age nine to 10, up until age 19 to 20.
The clinical data includes mental health measures of depression, anxiety and aggression, as well as reports from subjects and their parents of internalizing and externalizing symptoms. The massive study size will provide Manning sufficient evidence to thoroughly explore the relationship between brain development and mental health.
Manning will review the MRI scans and clinical data, and analyze them for biomarker patterns in brain structure and function. By correlating the biomarkers displayed in the MRI scans to mental health measures experienced by the subjects, she hopes to create a new set of diagnostic methods for clinicians and transform our understanding of the developmental brain features that support mental health in adolescence. This work will also enable Manning to identify what the baseline is for a child’s brain in these vulnerable regions of the brain, to help predict future mental health symptoms.
Dr. Matthias Wilms, PhD, Department of Radiology (Dr. Nils Forkert, PhD)
Matthias Wilms is focused on improving machine learning models used in brain age prediction and analysis, which can help clinicians diagnose and analyze neurodegenerative diseases like Alzheimer’s. Scientists like Wilms design machine learning models by “training” computer systems to learn patterns from a large, representative set of data, and then having the system use what it learned from that data to solve diagnostic or analysis tasks in neuroimaging..
Wilms is working to expand on traditional predictive machine learning models that analyze an MRI scan of the human brain to identify its biological brain age. Biological brain age prediction can help clinicians to identify patients with signs of brain degeneration early on.
Wilms is creating a method that combines predictive modelling with generative modelling — a model that works the other direction, looking at a brain in its current state and working backward to identify underlying, earlier biomarkers of brain degeneration. This combined method will use a new invertible type of machine learning, which can evaluate the brain in both directions at the same time — predicting a patient’s current brain age, and generating images of how the brain developed leading up to the scan and how it will continue to degenerate.
Wilms hypothesizes that the invertible method will provide more accurate, consistent, and interpretable models that will be sought after in both clinical and basic research settings, to improve the diagnostic process for patients with degenerative brain disorders.
This prestigious group represents the breadth and depth of the research and expertise at the University of Calgary, representing a number of the Institutes within the Cumming School of Medicine, including the Alberta Children’s Hospital Research Institute, the Owerko Centre, The Mathison Centre for Mental Health Research & Education at the Hotchkiss Brain Institute (HBI) and the HBI.